The activity has been taken from Chapter 1 Chemical Reactions and Equations
Displacement Reactions
Aim of the Activity
To
demonstrate the displacement reaction between iron and copper sulphate
Procedure
1) Take
three iron nails and clean them by rubbing them with sandpaper.
2) Take
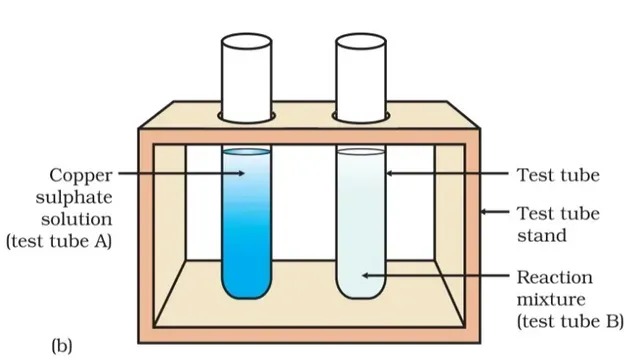
two test tubes marked as (A) and (B). In each test tube, take about 10 ml of copper sulphate solution.
3) Tie
two iron nails with a thread and immerse them carefully in the copper sulphate
solution in test tube B for about 20 minutes (fig). Keep one iron nail aside
for comparison.
4) After
20 minutes, take out the iron nails from the copper sulphate solution.
5) Compare the intensity of the blue colour of copper sulphate solutions in test tube (A) and (B).
6) Also,
compare the colour of the iron nails dipped in the copper sulphate solution
with the one kept aside.
.jpg)
Observation
After 20 minutes we take out both the nails from test tube B.
Now we compare both nails with the nail kept aside, and we find the iron nail that remained suspended has a brownish coating on its surface.
we also find that the blue colour of the copper sulphate solution fades and changes to light green colour which is different from the colour of the copper sulphate solution in test tube A.
Fe(s) + CuSO4(aq) → FeSO4(aq) + Cu(s)
(Copper sulphate) (Iron sulphate)
In the above reaction, iron displaces copper from the copper sulphate solution.
Conclusion
This activity shows the displacement of copper from copper sulphate by iron.
You can watch The video of this activity
Frequently Asked Questions
1. What is the colour of the copper sulphate solution?
Ans. Shiny blue colour
2. Why do iron nails become brown in colour?
Ans. A layer of copper is coated over iron nails when deposited in a copper sulphate solution.
3. What is the name of the substance (green colourd) formed?
Ans. Ferrous sulphate (FeSO4)
4. Write the definition of Displacement reaction’.
Ans. The reaction in which one atom or a group of atoms of one compound is displaced by one atom or a group of atoms.
5. Give the chemical equation of the reaction.
Ans. Fe + CuSO4 → FeSO4 + Cu
Related Topics for you
S.No. | Topic |
1 | |
2 | |
3 | |
4 | |
5 | |
6 | |
7 | |
8 | |
9 | |
10 | |
11 |

.jpg)



No comments:
Post a Comment