This activity explains the Photolysis of silver chloride in the presence of sunlight
Photolysis of Silver Chloride
Caution: This activity needs the teacher’s assistance.
Aim of Activity
To find the change in colour of silver chloride after decomposition
Procedure
1. Take about 2 g of silver chloride in a China dish.
2. What is its colour?
3. Place this China dish in sunlight for some time
4 . Observe the colour of the silver chloride after some time.
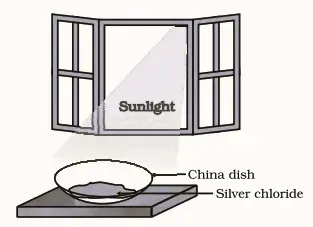 |
Observation
When silver chloride is placed in the sunlight. Colour of silver chloride changes.
Conclusion
On the decomposition of silver chloride which is white in colour, it changes to grey.
sunlight
2AgCI (s) → 2Ag(s) + CI2 (g)
(white) (grey)
Silver chloride decomposes into silver and chlorine.
so, the decomposition reaction takes place in the presence of sunlight, it is called Photolysis.
You can watch the video for this activity
Frequently asked questions
1. What is the colour of silver chloride?
Ans. Silver chloride is a white powder.
2. Name the reaction that takes place in this activity.
Ans. Photolysis
3. Name the substance formed after the decomposition of silver chloride.
Ans. Silver (Ag) and chlorine (Cl)
4. Write the definition of ‘Decomposition reaction’.
Ans. Complex substance breaks down into two or more simple substances in the presence of energy(heat, light, electricity )
5. Give the chemical equation of the reaction.
Ans. 2AgCl → 2Ag+ Cl2
Related Topics for you
S.No. | Topic |
1 | |
2 | |
3 | |
4 | |
5 | |
6 | |
7 | |
8 | |
9 | |
10 | |
11 |
.jpg)
.jpg)



No comments:
Post a Comment