Propanone is an organic compound having a Ketone group. Today we will discuss how to draw electron dot structure of propanone.
Propanone is also called Acetone.
We will discuss the electron dot structure of propanone through the following points
1. Formula
of propanone
2. Electronic
configuration of carbon
3. Electronic
configuration of hydrogen
4. Electronic
configuration of oxygen
5. Find
the central atom in the compound
6. Draw
the structural formula of propanone
7. Electron
dot structure of propanone
Formula of Propanone
`CH_3COCH_3` or `C_3H_6O`
Electronic configuration of carbon
`1s^2 2s^2 2p^2`
Carbon has four valence electrons in the outermost
shell.
Electronic configuration of hydrogen
`1s^1`
Hydrogen has one valence electron in the outermost
shell.
Electronic configuration of oxygen
`1s^2 2s^2 2p^4`
Oxygen has 6 valence electrons in the outermost shell.
Find the central atom in the compound
Propanone has three carbon atoms including -CO- group.
Carbon atoms form the main chain of the compound.
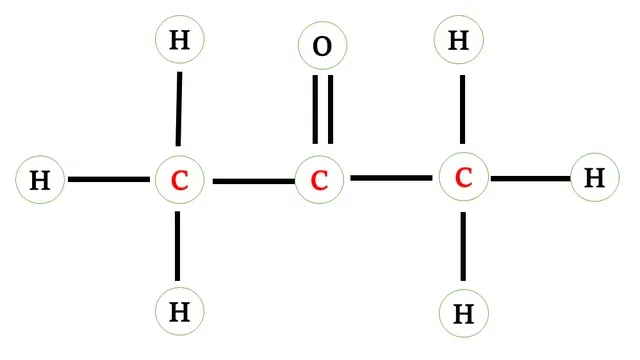
Structural formula of Propanone
The carbon atom requires four electrons to complete its
octet and hydrogen atom requires one more electron to attain stable noble gas
configuration and the oxygen atom requires two electrons.
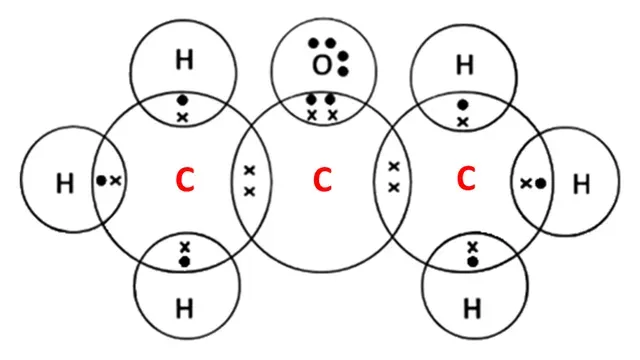
Two carbon atoms other than -CO- group share 1-1
electron with hydrogen atoms and carbon.
In the -CO- group, carbon and oxygen atoms share 2-2 electrons forming a double bond.
Electron dot structure of Propanone
Related Topics




No comments:
Post a Comment