Ethyne is an unsaturated hydrocarbon that belongs to the Alkyne series. You will learn the electron dot structure of ethyne.
We will discuss the electron dot structure of ethyne through the following points
Electron dot structure of C2H2
1. Formula of
ethyne
2. Electronic
configuration of carbon
3. Electronic
configuration of hydrogen
4. Find the
central atom in the compound
5. Draw the
structural formula of ethyne
6. Electron
dot structure of ethyne
Formula of ethyne
`C_2H_2`
Electronic configuration of carbon
`1s^2 2s^2 2p^2`
Carbon has four valence electrons in the outermost
shell.
Electronic configuration of hydrogen
`1s^1`
Hydrogen has one valence electron in the outermost
shell.
Find the central atom in the compound
There are two carbon atoms attached to two hydrogen
atoms, carbon atoms are the central atoms.
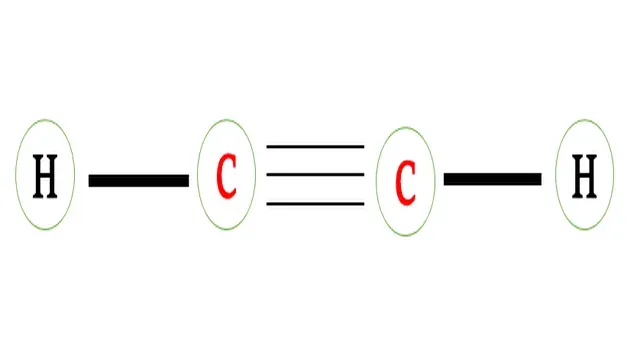
Structural formula of ethyne
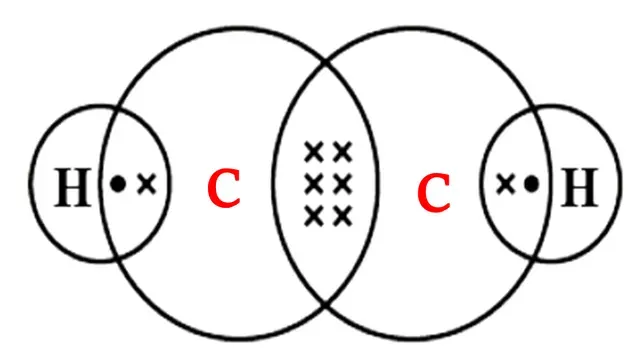
Carbon atom requires four electrons to complete its octet
and each hydrogen atom requires one electron to complete its octet.
Each carbon shares its one valence electron with one hydrogen atom, the remaining three valence electrons with another carbon atom and each hydrogen atom shares one valence
electron with carbon atom.
There is one triple bond between two carbon atoms and a Single bond (covalent bond) is present between carbon and hydrogen atoms.




No comments:
Post a Comment