Today we will learn What happens when lead nitrate is heated. The reaction is related to Chemical reactions and equations.
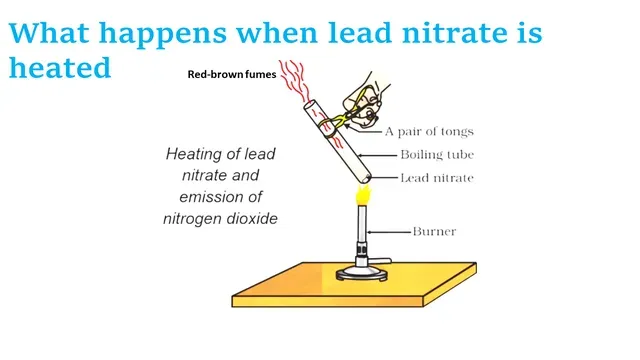
In the chemistry lab, with the teacher's assistance, we will do the experiment of heating lead nitrate.
Required material- Lead nitrate, dry test tube, and burner
Lead nitrate is a white powder and the lead nitrate formula is Pb(NO3)2.
When lead nitrate is heated,
it breaks down into lead oxide, nitrogen dioxide, and oxygen gas because the thermal
decomposition of lead nitrate takes place.
The chemical equation shows the heating
of lead nitrate and the emission of nitrogen dioxide and oxygen gases.
`Pb(NO_3)_2\overset{\Delta
}{\rightarrow}PbO+ NO_2\uparrow +O_2\uparrow`
Red-brown fumes are produced when
lead nitrate is heated, these fumes are of nitrogen dioxide gas
Heating of lead nitrate undergoes
thermal decomposition because by absorbing heat, lead nitrate breaks down into
simpler products like lead oxide, nitrogen dioxide, and oxygen.
As heat is absorbed during this
reaction so it is an endothermic reaction.
Precautions:- The thermal decomposition of lead nitrate can
release toxic lead oxide fumes and nitrogen dioxide gas. Therefore, it is
essential to carry out this reaction with proper safety precautions and under
proper ventilation.
Take a test of your knowledge
1. What happens when magnesium ribbon is burnt?
2. What happens when Zinc reacts with sulphuric acid?
3. What happens when barium chloride reacts with sodium sulphate?
4. What happens when copper is heated in the air?
5. What happens when a potassium iodide solution is added to a solution of lead nitrate?
6. What happens when silver chloride is exposed to sunlight?
7. What happens when iron nails are placed in copper sulphate?
FAQs
1. What is the formula of lead nitrate?
Ans.-Pb(NO3)2
2. Which reaction takes place when lead nitrate is heated?
Ans.- Thermal decomposition reaction takes place when lead nitrate is heated in a test tube.
3. Is heating lead nitrate an exothermic or endothermic reaction?
Ans.- As it undergoes thermal decomposition so it absorbs heat that’s why it is an endothermic reaction.
4. Which gases evolve when lead nitrate is heated?
Ans. – when lead nitrate is heated, two gases nitrogen dioxide and oxygen evolve.
5. What is the colour of nitrogen dioxide gas?
Ans.- Nitrogen dioxide gas evolve in the form of red-brown
fumes.




No comments:
Post a Comment