In this post, you will find NCERT Science Class 9 Chapter 2 Activity solutions. In Class 9 science chapter 2 Is Matter Around Us Pure consists of NCERT Activity 2.1 Class 9 Science Explanation with a conclusion.
Activity
2.1 NCERT Class9 Science Is Matter Around Us Pure
You are suggested to study NCERT activity 2.1 class 9 science Is matter around us pure explanation with a conclusion so that you can attempt questions based on the activity 1.14 class 9 science. Activity 2.1 class 10 science will be very useful for you.
Activity 2.1 Class 9 Science
·
Let us divide the class into groups A, B, C and D
·
Group A takes a beaker containing 50mL of water and one spatula full of
copper sulphate powder. Group B takes 50 mL of water and two spatulas full of
copper sulphate powder in a beaker.
·
Groups C and D can take different amounts of copper sulphate and
potassium permanganate or common salt(sodium chloride) and mix the given
components to form a mixture.
·
Report the observation of the uniformity in colour and texture.
·
Groups A and B have obtained a
mixture which has a uniform composition throughout. Such mixtures are –(i) salt
in water and (ii) sugar in water. Compare the colour of the solutions of the
two groups. Through, both the groups have obtained copper sulphate solution but
the intensity the colour of the solution is different. This shows that a
homogenous mixture can have a variable composition.
·
Groups C and D have obtained mixtures, which contain physically distinct
parts and have non-uniform composition. Such mixtures are called heterogeneous
mixtures. Mixtures of sodium chloride and iron filings, salt and sulphur and
oil and water are examples of heterogeneous mixtures.
Observation
Group A and B prepare a solution of copper sulphate with water. Group A
take one spatula of copper sulphate as solute whereas group B takes two spatulae
of copper sulphate as solute.
Copper sulphate dissolves in water uniformly and makes a homogenous solution
but the concentration of the solution is different in both cases so the colour of both
the solutions is different.
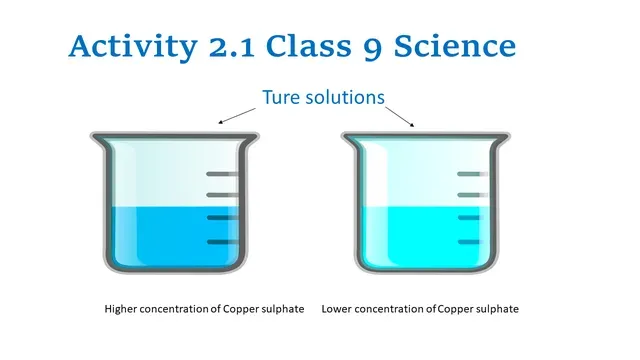 |
| True solutions |
Groups C and D mixtures of salt and sulphur or oil and water, in the mixture
particles of a substance, can be seen distinctly so the mixture is called heterogeneous.
Conclusion
Mixtures having uniform composition are homogenous and mixtures having
non-uniform mixtures are heterogeneous mixtures.
Some soluble substances like copper sulphate when dissolved in water
form homogenous mixtures whose composition depends upon the amount of
substance dissolved. On the other hand, substance which has a non-uniform
composition form heterogeneous
mixtures.
Related Topics for you
1. Activity 2.2 Class 9 Science
2. Activity 2.10
3. Solutions of Chapter 2 Is Matter Around Us Pure
.jpg)


