In this post, you will find NCERT Science Class 9 Chapter 1 Activity solutions. In Class 9 science chapter 1 matter in our surroundings consists NCERT Activity 1.13 Class 9 Science Explanation with a conclusion.
NCERT Activity 1.13 Class 9 Science Explanation with conclusion
You are suggested to study NCERT activity 1.13 class 9 science
explanation with conclusion so that you can attempt questions based on the
activity 1.13 class 9 science.
Activity 1.13 Class 9 Science
·
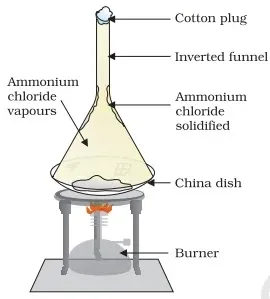
Take some camphor of ammonium chloride. Crush it and put it in a china
dish.
·
Put an inverted funnel over the china dish.
·
Put a cotton plug on the stem of the funnel.
·
Now heat slowly and observe.
·
What do you infer from the above activity?
 |
| sublimation |
Observation Activity 1.13 Class 9 Science
It is observed that camphor or ammonium chloride directly
gets converted into a gas without undergoing a liquid state. These vapours get
condensed on the upper part of the funnel which is cooler.
Impurities present in camphor are not volatile so they remain in a solid-state in the china dish.
The process in which a solid substance gets converted into a gaseous state
without undergoing a liquid state is called sublimation.
Conclusion Activity 1.13 Class 9 Science
When a substance is converted directly from solid to gaseous without undergoing a liquid state, then this phenomenon is called sublimation.
Querries based on Activity 1.13 Class 9 Science
1. What is sublimate?
Ans. The substance obtained by cooling the vapours is known as sublimate.
2. What is sublimation?
Ans. The change of solid directly into the gaseous state without undergoing the liquid state is known as sublimation.
3. Name some substances which show sublimation.
Ans. – Camphor, naphthalene, ammonium chloride
Related Topics
1. Next Activity- Activity 1.14 Class 9 Science
2. Previous Activity - Activity 1.12 Class 9 Science
.jpg)


