In this post, you will find NCERT Class 10 Science Activity 2.9 Explanation in detail. This activity is based on Chapter 2 Acids, Bases, and Salts of Class 10 Science.
NCERT Class 10 Science Activity 2.9 Explanation
Activity -Take
about 1g solid NaCI in a clean and dry test tube and set up the apparatus as
shown in ahead fig.
· Add
some concentrated sulphuric acid to the test tube.
· What
do you observe? Is there gas coming out of the delivery tube?
· Test
the gas evolved successively with dry and wet blue litmus paper.
· In
which case does the litmus paper change colour?
· On
the basis of the above activity, what do you infer about the acidic character
of:
(i)
dry HCI gas.
(ii)
HCI solution.
Note to teachers -
If the climate is very humid, you will have to pass the gas produced through a
guard tube (drying tube) containing calcium chloride to dry the gas.
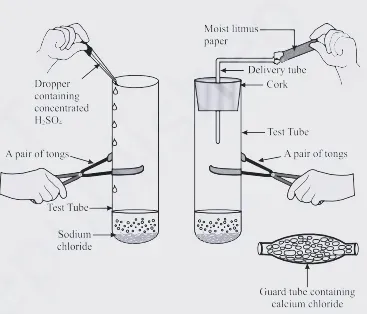 |
| Production of HCl gas |
Observation
Sulphuric acid reacts with sodium chloride to produce HCl gas and sodium sulphate.
2NaCl + H2SO4
→2HCl + Na2SO4
When we bring dry blue litmus
paper near the opening of the calcium chloride tube, the colour of the litmus paper
remains unchanged.
On bringing a moistened blue
litmus paper near the mouth of the delivery tube, the blue litmus changes to
red.
Explanation
Acids are substances that can release protons (H⁺ ions) when dissolved in
water.
Hydrochloric acid (HCl)gas produces hydrogen ions (H⁺) and chloride ions (Cl⁻)
in the case of wet blue litmus paper.
`HCl →H^+ + Cl^-`
Hydrogen ions react with water molecules and form hydronium ions.
`HCI(g)+
H_2O(l) → H_3O^+ + CI^-`
(H⁺) ions show an acidic character.
Conclusion
Dry HCl gas has no effect on
blue litmus paper. But in the presence of moisture, hydrogen chloride gas turns
blue litmus to red. Thus HCI gas shows its acidic character only in the
presence of water.
HCI(g)+ H2O(I) → H3O+ + CI-
Similarly, bases in the presence of water produce OH-
(aq) ions.
NH3(g) + H2O(I) →
NH4(aq) + OH-(aq)
Watch Video to understand the activity 2.9
Questions based on Activity 2.9
1. What is the formula of sulphuric acid?
Ans- H2SO4
2. Write the reaction between NaCl and sulphuric acid.
Ans - 2NaCl + H2SO4
→2HCl + Na2SO4
3. Which gas evolve during the activity 2.9 Class 10
Science?
Ans - HCl gas
4. What is the use of a calcium chloride (CaCl2) guard
tube?
Ans - Calcium chloride is a drying agent and a tube containing
CaCl2 makes the HCl gas dry in activity 2.9.
5. Which ion is responsible for the acidic character?
Ans- Hydrogen ions are responsible for acidic character.
Related Topics -
2. Activity 2.8
.jpg)


