Here you will find NCERT Activity 3.14 Class 10 Science Explanation that belongs to Chapter 3 Metals and Non-metals. After reading Solutions of chapter 3 Metals and Non-metals, you will be able to understand activity 3.14.
NCERT Activity 3.14 Class 10 Science Explanation
Activity 3.14 Class 10 Science
Object - To find the conditions that are
responsible for rusting
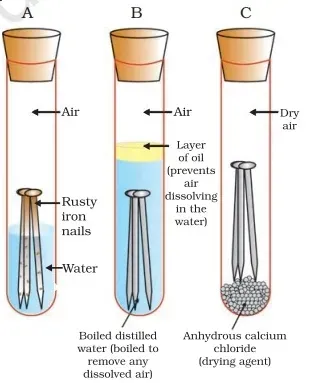
· Take
three test tubes and place clean iron on nails in each of them.
· Label
these test tubes A, B, and C. Pour some water into test tube A and cork it.
· Pour
boiled distilled water into test tube B, add about 1 ml of oil, and cork it. The
oil will float on water and prevent the air from dissolving in the water.
· Put
some anhydrous calcium chloride in test tube C and cork it. Anhydrous calcium
chloride will absorb the moisture, if any, from the air, Leave these test tubes
for a new day and then observe
Observations:
It is observed that iron nails rust in test tube A but they do not rust in test
tubes B and C. In test-tube A, the nails are exposed to both air and water. In
test-tube B, the nails are exposed to water only and the nails in test tube C
are exposed to dry air free from water vapour.
Conclusion: The presence of both air and moisture is essential for rusting to take place. Both air and water are present, B there is no air dissolved in the eater, and C
the air is dry.
Related Topics -
1. First activity - Activity 3.1
2. Previous activity -Activity 3.13
3. Solution of Chapter 3 Metals and Non-metals
5. Extra Questions of Chapter 3
6. Solutions of Class 10 Science all chapters
.jpg)


