Download the NCERT Solution for Class 9 Science Chapter 1 – Matter in our surroundings to help you to understand the concepts and basics. The NCERT Solution for Class 9 Science Chapter 1 – Matter in Our Surroundings can be downloaded in PDF format free of cost.
Matter in Our Surroundings class 9 explanation will help you to answer the following questions –
1. What is matter?
2. What are the main physical states of matter?
3. What are the main characteristics of particles of matter?
ii. The
particles are moving continuously.
iii. The
attraction force between the particles is weak.
iv. The
particles of matter are very small.
So study the chapter – Matter in Our Surroundings class 9 and answer the following questions-
5. Can the state of a matter be changed?
6. What is the effect of change in temperature on a matter?
7. What is evaporation?
8. Factors affecting rate of evaporation?
Here you will find
Matter in our surroundings class 9 extra questions.
NCERT Solutions for Class 9 Science Chapter 1Matter in our Surroundings
So first of all you must know the topics and subtopic of Chapter 1 Matter in our Surroundings of NCERT Science.
2. Characteristics of Particles of Matter
3. States of Matter
4. Can Matter Change its State?
5. Evaporation
Students of all Educational boards can
download Solutions of Chapter 1 Matter in our Surroundings of NCERT
Science for Class 9 in English medium and Hindi medium in PDF format for free.
Your problem of downloading ‘Matter in our surroundings class 9 notes’ has been
solved.
You can also watch videos of Solutions of
Chapter 1 Matter in our
Surroundings of NCERT Science for
Class 9 for online. The solution is based on the latest syllabus of CBSE 2021-22.
Solutions of Chapter 1 Matter in our
Surroundings of NCERT Science for
Class 9 Intext questions
Chapter
1 Class 9
Matter in our Surroundings
Intext Questions
From Page No. 3
Q1.Which of the following are matter?
Chair, air. Love, smell, hate, almonds, thought, cold, cold- drink, the smell of perfume.
Ans.Matter- Anything around us which occupies space has mass, and can be felt with our sense organs is called a matter. Following are the matter-
Chair,
Air, smell, almonds, cold drink, and smell of perfume
Q2. Give reason for the following
observation-
The smell of hot sizzling food reaches you several meters away, but to get the smell from cold food you have to go close.
Ans. The smell of food reaches us due to ‘Diffusion’. The rate of diffusion increases due to high temperature because the kinetic energy of particles increases with an increase in temperature. That’s why the smell of hot sizzling food reaches us several meters away and faster. But on the other hand smell of cold food does not get diffused so fast that’s why we have to go close to get its smell.
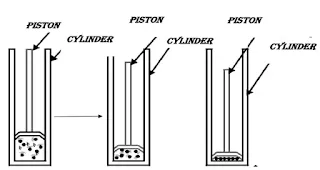
Q3.A driver is able to cut through water in a swimming pool. Which property of matter does this observation show?
Ans. The above activity explains the following properties of water (liquid state)
i. The inner particle force of attraction is weak
ii. Particles in a liquid have a larger space between them.
iii. Particles of liquids move easily.
Q4. What are the
characteristics of the particles of matter?
Ans.
i.The
particles have inter-particle space between them.
ii. The
particles are moving continuously.
iii. The
attraction force between the particles is weak.
iv. The particles of matter are very small.
From Page No. 6
Q.1The mass per
unit volume of a substance is called density (density = mass/volume)/. Arrange
the following in order of increasing density: air, exhaust from chimneys,
honey, water, chalk, cotton, and iron
Ans. The density of a substance depends upon the no. of particles per unit volume
as well as upon their mass. On the basis of these three factors, i.e., no. of particles,
size of particles and force of attraction the substances can be arranged in increasing
order of density as
follow-
Air < exhaust from chimneys <
cotton < water < honey < chalk < iron
Q.2 (a) Tabulate the differences in the characteristics of states of matter.
Ans
|
Properties
|
Solid
|
Liquid
|
Gas
|
|
Shape |
Definite
|
Indefinite
|
Indefinite
|
|
Volume
|
Definite
|
Definite
|
Indefinite
|
|
Inter-particle
attraction |
Strong
|
Lesser
than solids |
Very
weak |
|
Compressibility |
Negligible
|
Very
small |
High
|
|
Rigidity
|
Very
hard |
Less
rigid |
Compressibility |
|
Fluidity
|
No
fluidity |
Fluidity |
High
fluidity |
|
Diffusion
|
Negligible
|
Slow |
Very
fast |
|
Kinetic
energy |
Very
low |
Higher
than solid |
Very
high |
(b) (b)comment upon the following
An Rigidity, compressibility, fluidity, filling a gas container, shape, kinetic energy, and density
Ans.
(i)Rigidity – It is the tendency to maintain the shape when some outward
force is applied. The rigidity is highest in solid matter.
(ii)Compressibility – It is the
tendency to decrease the volume on the application of force. The matter has inter-molecular
space between them, when an external force is applied on them the particles come
closer, this is known as compressibility. The compressibility is highest in
gases because of the largest inter-particle spaces.
(iii)Fluidity – The tendency to flow is called fluidity. The
fluidity is highest in gases due to the very weak inter-particle force of
attraction. Liquids also have a tendency to flow but their fluidity is smaller
than gases due to comparatively smaller inter-particle distances.
(iv)Filling of a gas container – The
gas particles vibrate and moves in all directions so they fill the container
of all shape and size. The gas particles
occupy all space in the container.
(v)Kinetic energy- The energy possessed
by the particles due to their motion. In
gases, the inter-particle spaces are
large while the inter-particle force of attraction is small. Due to this reason,
the particles of gases move at high speed hence, have the highest kinetic energy. The kinetic energy of liquids is lesser than gases, while it is least in solids at
room temperature.
(vi)Density-It is the mass occupied by
a matter per unit volume. It can be obtained by dividing the mass of a
particular solid by the volume occupied by that mass of the solid.
(mass/volume)
(vii)Shape-The shape of the matter
depends on the inter-particle force of attraction and inter-particle spaces.
The solids have a fixed shape due to the strongest force of attraction and smallest
inter-particle spaces on the other hand liquids do not have a fixed shape due to the comparatively weaker force of attraction and larger inter-particle spaces.
Q.3 Give reasons:
(i) A gas completely fills the vessel in which it is kept?
Ans.
(i) The particles of gas move freely in all directions at different speeds. A gas in
the vessel completely fills it in which it is kept due to the phenomenon of diffusion.
In the gaseous state, the inter-particle spaces are very large hence, they readily fill the vessel completely due to their motion.
(ii) Gas exerts pressure on the walls of the container?
Ans.Force per unit, area is called pressure. The particles of gas moving with high speed strike the wall of the container and exerts force on it.
(iii)A wooden table
should be called a solid?
Ans. A wooden table should be called a solid because it has a definite shape, fixed
volume, incompressible nature, rigidity, and no movement of the constituent
particles present in them.
(iv)We can easily
move our hand in the air but to do so the same through a solid block of wood we
need a ‘karate expert’.
Ans. The inter-particle spaces in the air are very large while the inter-particle force of attraction is very weak. This is the reason that our hand can move, in the air. But the inter-particle spaces in solid are very small and the inter-particle force of attraction is very strong. So we cannot easily overcome but only a ‘karate expert’ with high power can separate the particles of solid.
Q.4Liquids generally have lower density as compared to solids but you must have observed that ice floats on water. Find out why?
Ans.Liquids have lower density as compared to solids. But the solid form of water like ice floats on the surface of liquid water. It indicates that the density of the solid form of water is lower than the liquid form of water. This is due to the open cage-like structure of ice. So there is some vacant spaces are left in ice Therefore due to larger vacant spaces the volume of ice increase hence, density decreases. That’s why it floats over the surface of the water.
From Page No. 9
Q.1Convert the
following temperature to Celsius scale.
(a) 300K (b) 573K
Ans.
The relation between Kelvin scale and
Celsius scale is as follows:
t(℃)=t(K)−273
(a). t(℃)=300−273=27℃
(b).t(℃)=573−273=300℃
Q.2 What is the physical state of water at?
(a) 2500C (b) 1000C
Ans.
(a) 250
(b) At 1000C, water starts boiling, so water exists both as a liquid as well as a gas.
Q.3For any substance, why does the temperature remain constant during the change of state?
Ans.When the change of state of substance beings, the energy which is now supplied is being used up as latent heat or hidden heat. Now the heat supplied is used up to overcome the inter-particle force of attraction. As a result, the temperature remains constant during the melting and boiling of a substance.
Q.4 Suggest a method to liquefy atmospheric gases?
Ans. Atmospheric gases can be liquefied either by decreasing temperature or by increasing pressure. During liquefaction of atmospheric gas, the constituent particles have to be brought closer to each other and this is done by cooling and applying pressure on it.
From Page No. 10
Q.1 Why does a desert cooler cool better on a hot dry day?
Ans. During hot and dry the temperature of the atmosphere is high and the humidity of air is low. The rate of evaporation increase as temperature increases and humidity decreases. So, desert cooler cools better on a hot dry day.
Q.2 How is the water kept in an earthen pot (matka) become cool during summer?
Ans. The water is kept in an earthen pot (Matka) become cool during summer due to
the following reasons:
i. In summer, the temperature is high so the rate of evaporation increases so, cooling increases.
ii. The particles of water with high kinetic energy escape through the pores of the earthen pot (matka) and evaporates, since evaporation causes cooling thus, the water cools.
Q.3 Why does our palm feel cold when we put some acetone or petrol or perfume on it?
Ans. Acetone or petrol or perfumes are liquids with low boiling points when they are put on the palm then they absorb heat energy of the palm and evaporate rapidly, so the palm feels cold.
Q.4 Why are we able to sip hot tea or milk faster from a saucer rather than a cup?
Ans. When the surface area increases then the rate of evaporation also increases so
When hot tea or milk is poured into a saucer that has a larger surface area than the surface area of a cup, the rate of evaporation increases, and tea or milk becomes a little cooler more quickly. Thus it becomes easier to sip hot tea or milk from a saucer rather than a cup.
Q.5 What type of clothes should we wear in summer?
Ans. We should wear light coloured cotton clothes because during summer we perspire more and cotton have a tendency to absorb sweat and allows the sweat to evaporate faster, this gives us a cooling effect in summer.
You will find NCERT Solutions of Class 10 Science
Chapter 1 Chemical Reactions and Equations
Chapter 2 Acids, Bases, and Salts
Chapter 3 Metals and Non-metals
Chapter 5 Periodic Classification of Elements
Chapter 7 Control and Coordination
Chapter 8 How Do Organisms Reproduce?
Chapter 16 Management of Natural Resources
NCERT EXERCISES
Q.1Convert the following temperatures to the Celsius scale:
(a) 293 K (b) 470 K
Ans. (a) 293 K
(a) 250 C (b)3730 C
Ans.
(a). 25
(b). 3730 C
(a) Naphthalene balls disappear with time without leaving any solid.
(b) We can get the smell of perfume sitting several meters away.
Ans.
(a) Naphthalene is a volatile solid which shows sublimation at room temperature.
As a result, it gets converted into a gaseous state. That’s why Naphthalene balls
disappear with time without leaving any solid.
(b)We can get the smell of perfumes sitting several meters away because perfumes have volatile substances and they diffuse faster in the air. During diffusion, the particles of the perfume mix with the particles of air and reach us sitting several meters away.
Q.4 Arrange the following substances in increasing order of forces of attraction
between the particles- water, sugar, oxygen.
Ans. We know that order of force of attraction between the constituent particles is as
follows
Solid > liquid > gas
Therefore, the force of attraction between particles in increasing order is as follows
Sugar > water > oxygen
Q.5 What is the physical state of water at?
(a) 250 C (b) 00 C (c) 1000 C
Ans.
(a) At 250 C, the physical state of water is a liquid.
(b) At 00 C the physical state of water can be either a solid (ice) or a liq
(c) At 1000 C the physical state of water can be either a liquid or a gas (steam).
Q.6 Give two reasons temperature is liquid.
(a) Water at room temperature is liquid.
(b)An iron almirah is a solid at room temperature.
Ans.
(a) Water is liquid at room temperature due to the following reasons
(i) Water takes the shape of the vessel in which it is kept. So water does not have a fixed shape, so it is a liquid.
(ii)The freezing point of water is 0
(iii)Water can be poured from one vessel into another vessel. So water has fluidity i.e. it can flow like a liquid
(b) An iron almirah is a solid at room temperature due to the following reason
(i)An iron almirah is hard and incompressible in nature that is, it has a fixed shape, so it is a solid
(ii). The melting point of iron is very higher than room temperature that’s why it is sol
(iii) An iron almirah cannot diffuse on itself through air i.e., it can not diffuse so it is solid.
Q.7 Why is ice at 273K more effective in cooling than water at the same temperature?
Ans. Ice (solid state of water) has extra energy in the form of latent heat of fusion
as compared to water (liquid).so ice absorbs more energy from surrounding
whereas water does not absorb energy at the same temperature, Thus, ice at
273K causes more cooling than water at the same temperature.
Q.8What produces more severe burns, boiling water or steam?
Ans. Steam at 373K (100
from surroundings and boiling water does not have latent heat Thus, steam
at 373K (1000C) have more energy than water at the same temperature and hence,
steam produces more severe burns than boiling water.
Q.9 Name A, B, C, D, E and F in the following diagram showing changes in its state: 
Ans.
B = Vaporization
C = condensation (Liquefaction)
D = Solidification (Freezing)
E = Sublimation
F = Sublimation (Solidification of gaseous state)
These NCERT solutions and study material will help you good marks for your CBSE Board and Other state board exams.
Remedial Education Point.com provides you complete study material for class 9 absolutely free. Now you can get accurate NCERT Book Solutions for Class 9 Science Chapter 1 Matter in Our surroundings notes prepared by our expert teachers.
NCERT Solutions for Class 9 Science All Chapters below
Chapter 5 The Fundamental Unit of Life

.jpg)



No comments:
Post a Comment